Current Research
Research in the Grillo Lab focuses on the molecular understanding of how genetic, age-related, or environmental-induced mitochondrial dysfunction alters metabolism through molecular oxygen pathogenicity and metal-mediated proteostatic failure in neurologic disease. Our research at the interface of biochemistry, metabolic physiology, and medicine employs multiple in vitro and in vivo models to investigate the basic biochemistry and molecular mechanisms of inborn errors of metabolism and age progressive neurodegenerative diseases.
|
Age is the greatest risk factor in acquiring most mortality causing diseases such as Alzheimer's Disease, heart disease, and diabetes. Mitochondrial dysfunction through deactivation of Complex I of the mitochondrial Electron Transport Chain remains one of the earliest features in these diseases. However, there remains an urgent need to better understand the influence of mitochondrial dysfunction on the molecular determinants that elicit biologic decline. Age-related reductions in ETC activity cause metabolic O2 imbalances, microvascular aging, and neuroinflammation in dementia and movement disorders. Our lab probes the etiological implications of mitochondrial dysfunction in proteotoxicity and illuminate the molecular underpinnings of Complex I deficiencies in neurometabolic diseases. To achieve this, we use a variety of cellular and animal models with the goal of discovering novel pharmaceutical interventions to treat biological decline with normative aging.
|
|
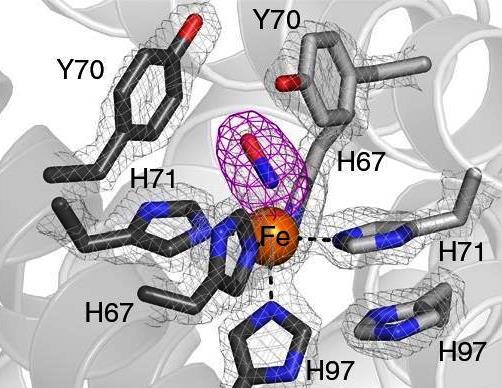
Mitochondrial dysfunction with age promotes metal deposition and reduces oxygen utilization, which may contribute to the oxidative stress that accompanies normative aging. Oxidative damage is widely implicated in explaining disease pathophysiology, however, there is a dire need to better understand the underlying molecular chain of events that cause this oxidative damage. Our lab investigates the pathophysiology of divalent metal and O2-mediated oxidative damage caused by mitochondrial dysfunction by identifying oxidized proteins that accumulate with age, determine which sites are vulnerable to metal-mediated oxidation, elucidate the fundamental biochemical principles that dictate metal binding, and probe their importance in biologic decline in normative aging. More broadly, we aim to unravel the largely uncharted metalloproteome, and uncover how O2 and metal imbalances influence the ~30% of the proteome that is predicted to bind metals.
|
|
There is a longstanding interest to understand the regulation of mitochondrial metabolism and the cross-talk between these complex pathways. Furthermore, there is a dire need to better understand alterations in metabolism in disease. Branched chain amino acid (BCAA) accumulation is implicated in age-related diseases (e.g. diabetes) and inborn errors of metabolism (e.g. maple syrup urine disease). Improper BCAA catabolism in mitochondria initiates early-onset muscle wasting and progressive brain degeneration in mice and patients. Severe nutritional BCAA restriction helps manage this disease, but there is a marked paucity of effective pharmacological interventions. Our lab aims to better understand the pathobiology of diseases associated with defective branched chain amino acid metabolism in mitochondria. This mechanistic-based approach will further enable the discovery of novel treatments for diseases such as Maple Syrup Urine Disease.
|